Continuous Glucose Monitoring System With Tubeless Insulin Pump
3:17
Insulet Corporation
A new technology for people with Type 1 diabetes, one that has been nearly a decade in the making, has been approved by the U.S. Food and Drug Administration.
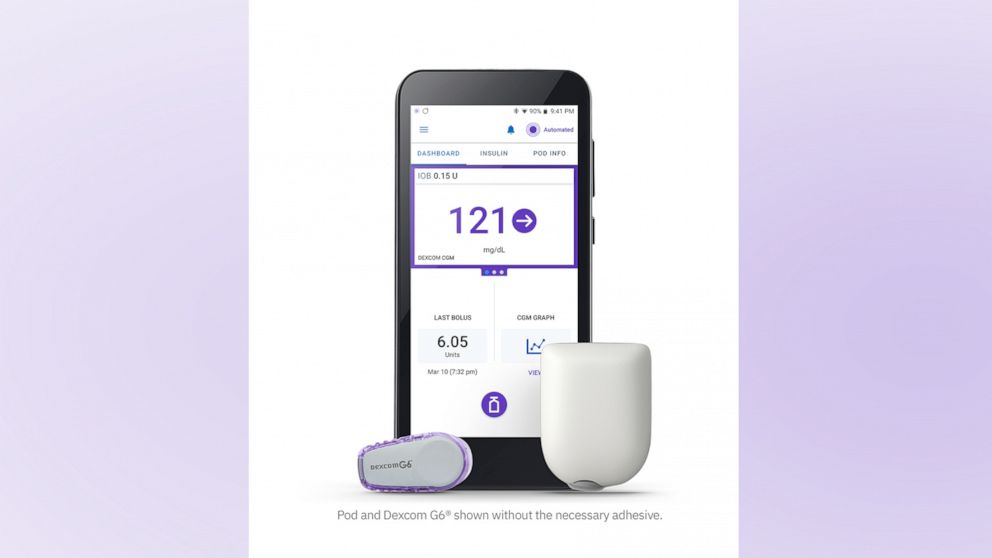
The Omnipod 5, which had been under FDA review since last year, is a hybrid, closed-loop insulin delivery system, meaning its insulin pump communicates directly with a user's continuous glucose monitor, in this case the Dexcom G6, to determine how much insulin to safely deliver, serving as almost an artificial pancreas.
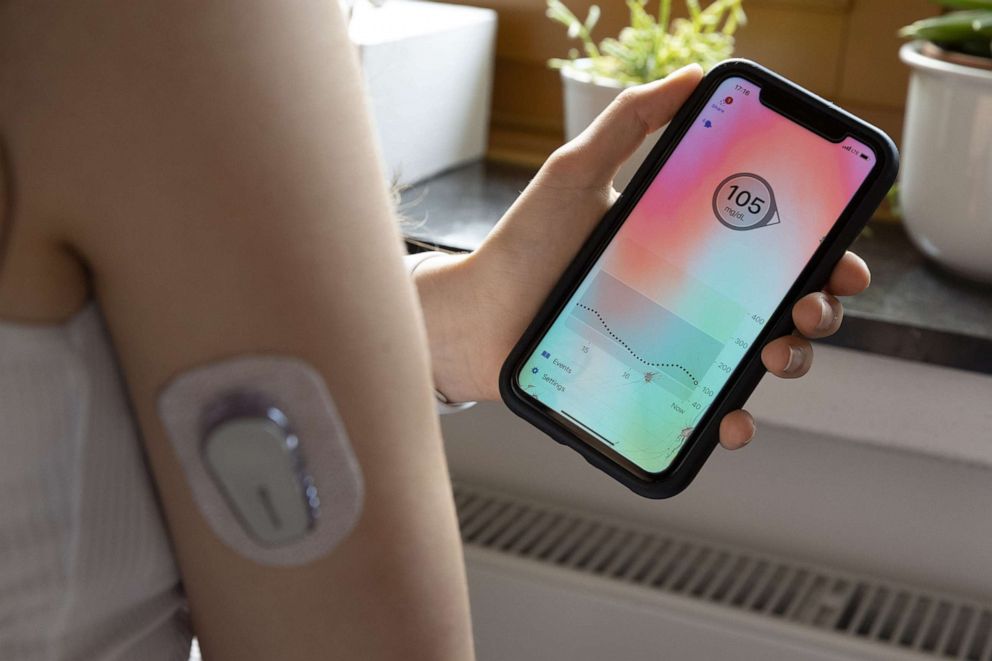
The Omnipod 5 is now the first tubeless system of its kind on the market and the first to be fully controlled via a smartphone app, eliminating the need to carry a separate device. It delivers insulin through a pod, smaller than the size of a desktop mouse, that the user manually fills with insulin, adheres to their skin, typically on their stomach, and replaces every three days.
"It is a remarkable technology that can dramatically improve the lives of people living with Type 1 diabetes," Shacey Petrovic, president and CEO of Insulet, the Massachusetts-based maker of Omnipod, told "Good Morning America." "Omnipod 5 helps to protect against highs and lows [blood glucose levels] during the day and at night to simplify life for people with diabetes."
The buildup of blood sugar in the body caused by a lack of insulin can lead to serious, long-term complications including blindness, increased risk of nerve damage, kidney disease, cardiovascular disease and stroke.
In addition to taking insulin, people with Type 1 diabetes must measure their blood glucose levels multiple times a day, by either finger pricks or wearing a continuous glucose monitor, a sensor that sticks on the skin and, through a wire that sits under the skin, measures blood sugar levels continuously.
For Gracie Brown, a 14-year-old from Tennessee, those daily burdens have weighed on her since the age of 4, when she was diagnosed with Type 1 diabetes.
Gracie, an eighth grade student who loves dancing and rowing and hiking, described living with diabetes as having a "constant calculator" in her mind trying to figure out dosing her insulin based on her blood sugar levels, food and activity and stress levels, all while trying to avoid both hypoglycemia, or low blood sugar, and diabetic ketoacidosis, dangerously high blood sugar.
For her mom, Hannah Brown, it is a constant worry about both the future health complications Gracie could face and the day-to-day struggles.
There are days that you just want to scream.
"When they told me my child had diabetes, my mind automatically went to 60 years from now. Is she still going to be able to see? Will she have her toes? Will her kidneys work?" said Brown. "I completely skipped over the 'let's take care of right now,' and I'm like, 'How do we take care of this forever?'"
"As that kind of settled in, and we learned how to navigate life with it, honestly it is a roller coaster," she said. "There are great days. There are awesome days. And there are days that you just want to scream."
Gracie was one of over 200 people to take part in the clinical trial for Omnipod 5 last year. Both she and her mom described the device as a game-changer.
"The closed loop system really makes your day more normal," said Gracie. "I'm not doing a constant calculation of, 'If I eat this now, what is my blood sugar going to be in third period when I have that big test?' or 'If I dose this much for my meal, am I going to be able to do our mile test in PE?'"
Brown said that when Gracie relied on an insulin pump alone, she was constantly "fixated" on her blood sugar levels and concerned about her falling too high or too low, both of which come with complications.
Since Gracie started using the Omnipod 5, Brown said, "Now we're not fixated on what Gracie's numbers are doing. We just let the system work."
A clinical trial success
People like Gracie who participated in the clinical trial for the Omnipod 5 used the system over a period of three months.
The trial results, released last March, showed that users had fewer episodes of hypoglycemia, were more in range with their blood glucose levels -- so had fewer extreme high and low blood sugar levels -- and lowered their HbA1c levels, an indicator of long-term glycemic control and an important data point for people with Type 1.
"The data was compelling and we really achieved the trifecta of clinical outcomes for people with diabetes," said Petrovic, who added that she was especially proud of the feedback from trial participants that the system was as easy to use as it was life-changing.
"We had many parents who said that they could go back to work for the first time since helping their child manage their Type 1 diabetes because they felt like they could trust the system to do that," said Petrovic. "The stories are endless and really powerful because it's a reminder of just how burdensome the condition can be."
It's a reminder of just how burdensome the condition can be.
The Omnipod 5 will quickly begin rolling out in a limited market release, to ensure a smooth delivery and training process, with a full release coming later this year, according to Petrovic. She said the device, which will be sold in pharmacies, will be immediately available for people ages 6 to 70, with clearance for kids ages 2 and older expected later this year.
The Omnipod 5 joins two other FDA-approved automated insulin delivery systems on the market, the Medtronic MiniMed 670G and Control-IQ from Tandem Diabetes Care, both of which require tubing to connect the insulin pump to the body.
What new technology means for people with Type 1 diabetes
More than 1.5 million people in the U.S. have Type 1 diabetes, which affects people of all ages.
In addition to excessive thirst, frequent urination and unexplained weight loss, symptoms of Type 1 diabetes can include blurry vision, nausea and stomach pains, fatigue and weakness and increased appetite, according to the Centers for Disease Control and Prevention.
Unlike the more common Type 2 diabetes, which is brought on by lifestyle factors and genetics, there is no known way to cure or prevent Type 1 diabetes, according to the CDC.
Advancements toward a cure like the one that made headlines in December -- when a man in Ohio with Type 1 diabetes received an infusion of new cells that restored his body's natural ability to create and regulate insulin -- are still years away from becoming widely available, experts say.
In the meantime, people with Type 1 diabetes must depend on new technologies that make their diabetes more manageable and in control.
"Over the last 40 years, insulin pumps have advanced to where they have been made smaller and they have been made smarter," said Dr. Lori Laffel, chief of the Joslin Clinic Pediatric, Adolescent and Young Adult Section at Joslin Diabetes Center and a professor at Harvard Medical School. "Now that we can get continuous glucose data, it allowed diabetes researchers to put together a way to send that glucose data to an insulin pump that was equipped with an algorithm that can direct the insulin pump to give more insulin if the glucose was high or rising or to give less insulin if the glucose was dropping or low."
Laffel, who was an independent investigator in the Omnipod 5 clinical trial, described the resulting hybrid, closed-loop insulin systems as "remarkable" feats.
"It is extraordinary what these systems have done," she said. "They have demonstrated that diabetes control is far improved without increasing the risk for low blood sugar levels, while at the same time reducing self-care burdens that can be very troublesome or bothersome to people of all ages living with diabetes, as well as family members, because diabetes is 24/7, 365 days a year."
Brown said the advancements she's seen in just the past decade Gracie has had Type 1 diabetes, culminating in the Omnipod 5, have given her daughter her childhood back.
"It makes me smile to see her be carefree with her friends, to see her be a teenager, to be goofy and to be silly and to not be so serious and concerned with what her health is doing this minute and not have to think hours down the road where she's at," said Brown. "That's what you want for your kid, to be a kid while they can be a kid, and I feel like we finally achieved that."
Source: https://www.goodmorningamerica.com/wellness/story/fda-approves-1st-automated-tubeless-insulin-pump-people-82402529


0 Response to "Continuous Glucose Monitoring System With Tubeless Insulin Pump"
Post a Comment